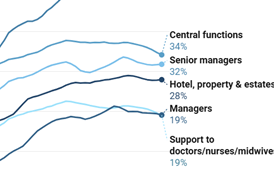
Analysed: drug pricing scheme promises better access to new medicines

Commissioners have been told they will gain easier access to the latest drugs approved by the National Institute for Health and Care Excellence after the Department of Health reached a deal on pricing with the pharmaceutical industry.
You need to be a subscriber to read more
Subscribe for unlimited access
With a HSJ subscription you’ll unlock:
- All HSJ news by sector, topic & region
- Breaking News announcements
- App for mobile and offline reading
- Comment and Daily Insights newsletters
- Regional roundup newsletters
- Unrestricted access to ‘Ask HSJ’ - AI assistant - AI assistant
- 10 expert briefings every fortnight (Premium only)
Already a subscriber? Sign into your account here