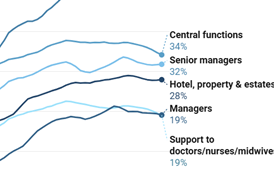
Round table - clinical priorities: a dose of realism
Wonder drugs will play a part in transforming healthcare - but a roundtable of experts brought together by HSJ and the Association of the British Pharmaceutical Industry said implementing best practice could be even more important. Alexis Nolan reports
You need to be a subscriber to read more
Subscribe for unlimited access
With a HSJ subscription you’ll unlock:
- All HSJ news by sector, topic & region
- Breaking News announcements
- App for mobile and offline reading
- Comment and Daily Insights newsletters
- Regional roundup newsletters
- Unrestricted access to ‘Ask HSJ’ - AI assistant - AI assistant
- 10 expert briefings every fortnight (Premium only)
Already a subscriber? Sign into your account here