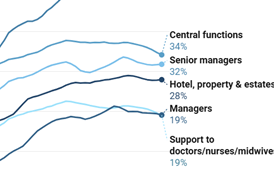
Medicines agency reports progress on over-the-counter medicines
Good progress is being made on the better regulation of over-the-counter medicines initiatives, the Medicines and Healthcare Products Regulatory Agency has found. In its second report on the initiative, the agency found developments include rollout of a self-certification scheme for prescriptions-only medicine and a review ...
You need to be a subscriber to read more
Subscribe for unlimited access
With a HSJ subscription you’ll unlock:
- All HSJ news by sector, topic & region
- Breaking News announcements
- App for mobile and offline reading
- Comment and Daily Insights newsletters
- Regional roundup newsletters
- Unrestricted access to ‘Ask HSJ’ - AI assistant - AI assistant
- 10 expert briefings every fortnight (Premium only)
Already a subscriber? Sign into your account here